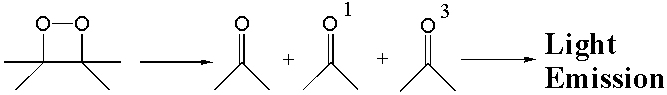
Chemiluminescence of lanthanide beta-diketonates in the reaction with 1,2-dioxetane
Model Description | Results | References
Introduction
The chemiluminescence of trivalent lanthanide(III)ions is observed from their inorganic, organic, and organometallic compounds in the following reactions:
1. The lanthanide ion accepts the energy of the excited product(s) of a chemiluminescent reaction and re-emits the chemiluminescence, but does not alter the rate or pathway of the reaction. The highly luminescent lanthanide chelates are employed to enhance the light intensity in those chemiluminescent reactions where the primary excited product is non-luminescent but can transfer its energy to the lanthanide chelate, for example, in the case of triplet excited species.
2. The lanthanide ion accelerates the rate of the chemiluminescent reaction, accepts the energy of the excited products, and emits the chemiluminescence, for example, in the chemiluminescent decomposition of 1,2-dioxetanes catalysed by lanthanide shift-reagents Ln(FOD)3 and Ln(DPM)3. The higher rate of the reaction results in the higher intensity of chemiluminescence.
3. The lanthanide ion initiates chemiluminescent reaction without being the emitter in it, for example, in the case of chemiluminescence observed in the oxidation of organic substances by cerium(IV).
4. The chemiluminescence of lanthanide ion is excited in redox transitions from the less-common oxidation states of the lanthanides, for example, in the reduction of terbium(IV) or praseodymium(IV), or the oxidation of europium(II) or ytterbium(II).
References on lanthanide chemiluminescence and triboluminescence
See recent review: "Chemiluminescence of systems containing lanthanide ions" by M. Elbanowski, B. Makowska, K. Staninski, M. Kaczmarek in J. Photochem. Photobiol. A: Chem. 2000, 130, 75-81.
For more information on chemi- and
bioluminescence, visit "The Society
for Bioluminescence and Chemiluminescence".
1,2-Dioxetanes are four-membered cyclic peroxides that easily decompose upon thermolysis to give the ketones in the ground state and in the singlet and triplet excited states. The decomposition of 1,2-dioxetanes is often accompanied by chemiluminescence due to the radiative deactivation of the ketone in the excited state.
The1,2-dioxetanes were extensively studied because they were found to be the key intermediates in bioluminescent reactions, and because they were shown to be excellent chemiluminescent labels in analytical biochemistry.
The intensity of chemiluminescence of the 1,2-dioxetanes can be substantially increased with the use of activators. The ideal activator accepts the electronic energy of both the singlet and triplet excited ketones, posesses high luminescence quantum yield, and re-emits the chemiluminescence in the desired spectral range. The organic dyes and the complexes of ruthenium and the lanthanides were used as the activators of 1,2-dioxetane chemiluminescence.
We have studied the system of Lanthanide Beta-Diketonate and Adamantylideneadamantane-1,2-Dioxetane (AAD).
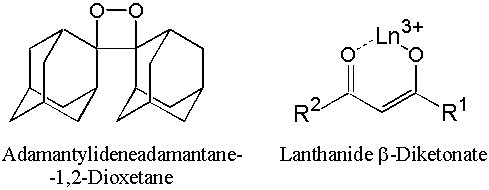
The lanthanide beta-diketonates were chosen as the activators because they show efficient, line-like luminescence of the lanthanide(III) ion in the visible and infrared spectral ranges. The 1,2-dioxetane AAD was chosen because it is exceptionally stable: its decomposition is accompanied by blue chemiluminescence with a maximum at 420 nm from the fluorescence of adamantanone.
1. Chemiluminescence of weakly emissive lanthanide ions praseodymium, neodymium and ytterbium
Previously, the lanthanide chemiluminescence was investigated mainly for europium or terbium compounds, whcih exhibit efficient visible luminescence (red and green, respectively).
A: We have observed the visible and infra-red chemiluminescence of praseodymium(III). The striking feature of the spectrum (shown below) is that the praseodymium emits with comparable efficiency from three excited ff-states, that is, the Vavilov's law is not applicable to this ion.
J. Photochem. Photobiol. A: Chem. 1998, 119, 177-186
Mendeleev Commun. 1998, 110-112
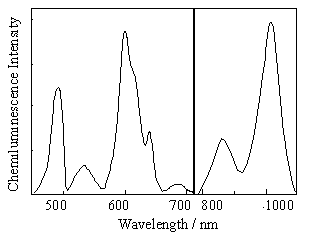
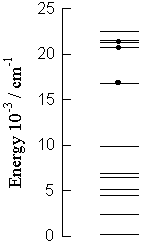
On the left: The chemiluminescence spectrum of Pr(FOD)3 in the reaction with 1,2-dioxetane AAD at 90 C in toluene. The emission bands of prasedoymium(III) are line-like and cover wide spectral range. The blue, yellow, and red emission bands are the transitions from the upper excited states of prasedoymium, while the red and infrared emission bands are from its lower excited state.
On the right: The excited states of prasedoymium(III). The emission of prasedoymium is observed from the three marked excited states. According to the Vavilov's law, the prasedoymium would be expected to emit only from the lower marked excited state, however, this is not the case.
B: We have studied the infra-red chemiluminescence of ytterbium(III) and neodymium(III) ions. The organic chelates of these near-infrared emitters give new opportunities to develop (chemi)luminescent lanthanide probes for analytical biochemistry.
J. Photochem. Photobiol. A: Chem. 2000, 131, 61-65
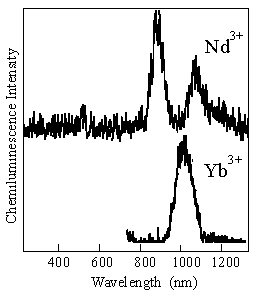
Figure: The chemiluminescence spectra of ytterbium(III) and neodymium(III) beta-diketonates during the decomposition of 1,2-dioxetane AAD at 90 C in toluene.
2. Singlet-singlet energy transfer from the ketone (adamantanone) to the singlet state of the the beta-diketone ligand in lanthanide beta-diketonates revealed by chemiluminescence
J. Photochem. Photobiol. A: Chem. 2000, 131, 61-65
We have observed that the intensity of blue chemiluminescence of adamantanone is quenched when a lanthanide beta-diketonate is added to the solution. The extent of chemiluminescence quenching by the chelate depends only on the type of the beta-diketone ligand; it is independent of the lanthanide ion; and it is proportional to the spectral overlap between the ligand-centered absorption of the chelate and the chemiluminescence spectrum of adamantanone.
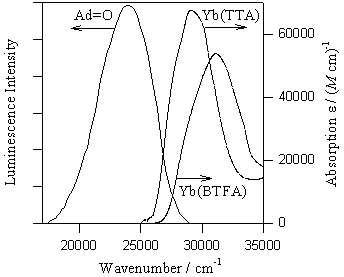
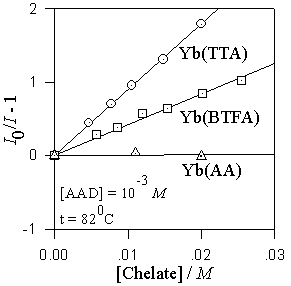
On the left: The chemiluminescence spectrum of adamantanone during the decomposition of 1,2-dioxetane in acetonitrile. The ligand-centered absorption spectra of ytterbium(III) beta-diketonates Yb(TTA)3.2H2O and Yb(BTFA)3.2H2O in acetonitrile. The ligand-centered absorption spectrum of Yb(AA)3.3H2O (not shown) is found above 30000 cm(-1). The spectral overlap for the chelates decreases in the order TTA > BTFA >> AA.
On the right: The quenching of the adamantanone chemiluminescence by the ytterbium(III) beta-diketonates Yb(TTA)3.2H2O, Yb(BTFA)3.2H2O, and Yb(AA)3.3H2O. Note that the efficiency of quenching by the chelates TTA > BTFA >> AA is directly proportional to the spectral overlap.
The first excited singlet state of adamantanone acts as the energy donor, while the excited singlet state of the ligand, which is responsible for the intense ligand-centered absorption of the chelates, acts as the energy acceptor. The observed quenching of the chemiluminescence results from the singlet-singlet energy transfer from adamantanone to the ligand in the chelate. For example, no quenching is observed with the AA chelates, while with the TTA chelates the kq approaches the diffusion limit.
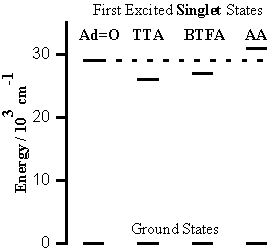
Figure: The first excited singlet states of adamantanone and of the beta-diketone ligand in the lanthanide chelates.
3. Enhancement of chemiluminescence of 1,2-dioxetane by various lanthanide ions
J. Photochem. Photobiol. A: Chem. 2000, 136, 203-208
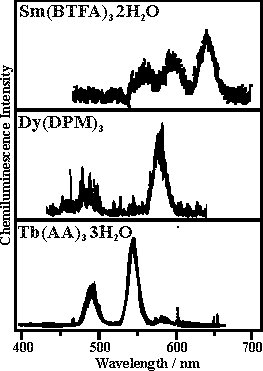
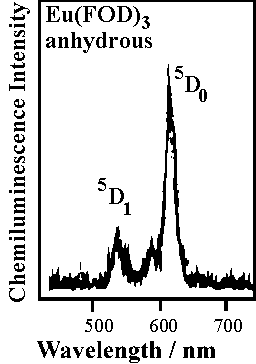
Figures: The chemiluminescence spectra of samarium(III), dysprosium(III), terbium(III), and europium(III) chelates excited during the decomposition of 1,2-dioxetane AAD in toluene at 90 C. All of the emission comes from the ff-transitions of the lanthanides(III). Note that for Eu(FOD)3, the emission is observed from two excited states: the lower-lying (5)D(0) and the upper-lying (5)D(1).
4. Catalytic decomposition of 1,2-dioxetane in the presence of lanthanide beta-diketonates
J. Photochem. Photobiol. A: Chem. 2000, 136, 203-208
J. Photochem. Photobiol. A: Chem. 1998, 119, 177-186
The coordination-unsaturated lanthanide(III) beta-diketonates catalyze the decomposition of 1,2-dioxetanes through the formation of a complex, in which the peroxide is coordinated to the lanthanide. The lanthanide(III) ion is excited by the intramolecular energy transfer from the carbonyl products formed when 1,2-dioxetane decomposes in the inner-coordination sphere of the lanthanide.
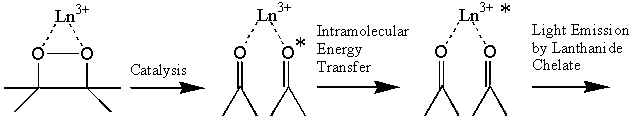
We have shown that those lanthanide chelates that catalyse the decomposition of 1,2-dioxetane exhibit higher intensity of the chemiluminescence than do those chelates that act only as energy acceptors.